Upcoming Events
See All Events
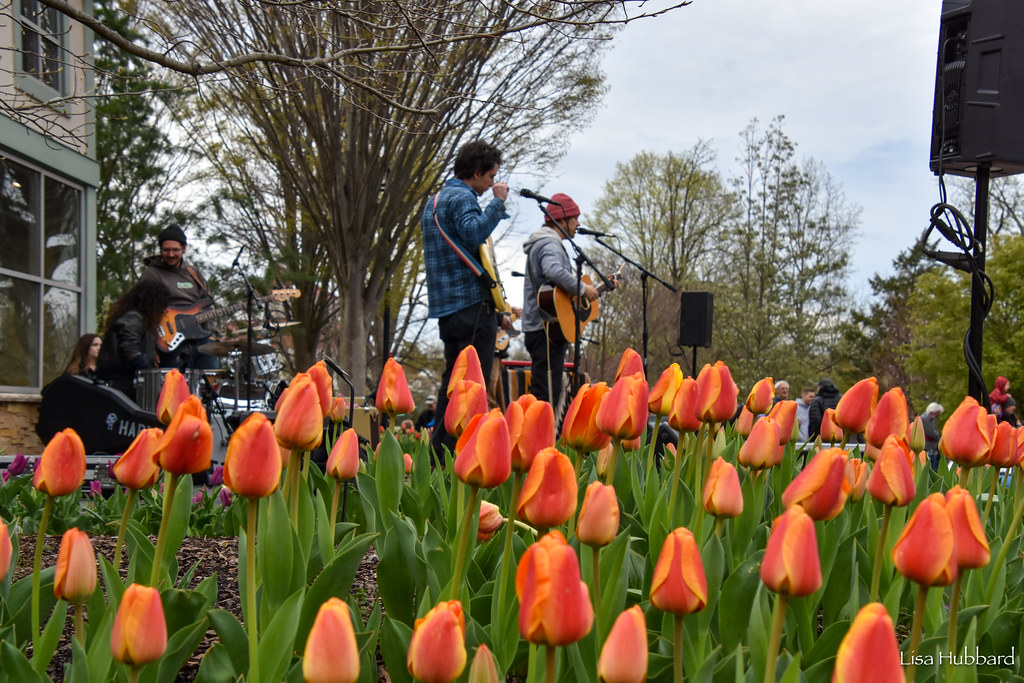
Tunes & Blooms

 Membership
Membership
Join the Zoo Family! As a member of the Cincinnati Zoo & Botanical Garden, you have year-round access and can visit as many times as you like! There’s something for everyone here. Come for a walk in the beautiful gardens, visit your favorite animals, or ride the new electric train! Members enjoy early entry, discounts on programs and in shops and restaurants, and free parking!


 Meet Fiona
Meet Fiona
Fiona is famous for surviving! She was born 6 weeks before she was due and was too small to stand and nurse from her mom, Bibi. The Zoo’s care team had to step in and figure out how to feed her, what to feed her, how to keep her warm, and how to manage critical care 24/7 while juggling other daily responsibilities.
Learn MoreMeet the Animals

Hippopotamus
Hippopotamus amphibius
Hippos can open their mouths about three times wider than people can.
Learn More
Red Panda
Ailurus fulgens
Red pandas spend up to 13 hours a day foraging for bamboo, eating only the youngest, most tender leaves.
Learn More
Hamerkop
Scopus umbretta
The hamerkop is named for how the shape of its heavy bill and large head crest resembles a hammer.
Learn More
Hellbender
Cryptobranchus alleganiensis
The hellbender is the largest fully aquatic salamander in the United States.
Learn More
 Conservation
Conservation
The Cincinnati Zoo, an accredited member of the Association of Zoos & Aquariums (AZA) since 1978, is internationally known for its success in the protection and propagation of endangered animals and plants and engages in research and conservation projects worldwide.






